January 23rd, 2024
The Difference Between Screening and Enrollment in Clinical Trials
By OneStudyTeam
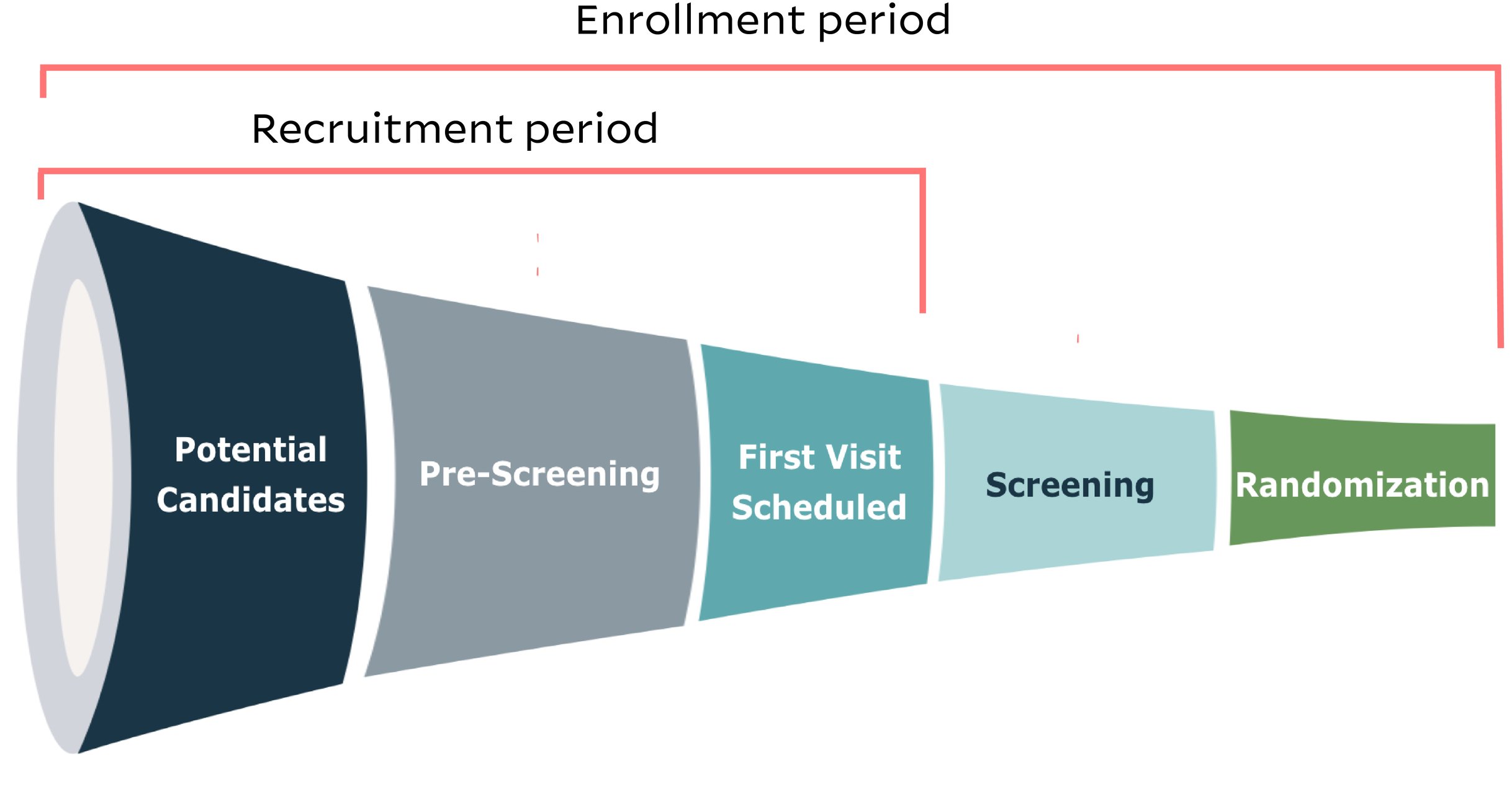
Clinical trial screening is one piece of the overall patient enrollment period. The screening phase immediately follows the recruitment period, during which a potential candidate has gone through pre-screening, has gotten their first visit scheduled with the research team and has learned more about the trial and about informed consent. Now, screening stands between that trial candidate and official enrollment. But what is the difference between screening and enrollment in clinical trials?
What happens during clinical trial screening?
Screening visits require a deeper assessment of whether a patient meets the inclusion/exclusion criteria in a protocol. Before screening can begin, the patient needs to sign the informed consent form to signify that they understand the study’s purpose, benefits, and risks, and they are voluntarily choosing to participate in the trial.
Once screening begins, the patient generally undergoes a series of evaluations, including a physical examination as well as any necessary lab tests or diagnostic tests such as blood tests and imaging. Screening ensures the patient is eligible to receive the investigational product safely and in a way that produces meaningful data to help researchers understand the efficacy of the product.
When screening a patient, the research site also collects baseline data that can function as a reference point when assessing changes as each patient moves forward in the trial. Baseline data can include medical history and health parameters.
How is a patient officially enrolled in a clinical trial?
A patient enrolls in the clinical trial once they have successfully undergone screening, and once the study team confirms that the patient has met all inclusion/exclusion criteria. Usually, the site team will verbally confirm with the patient that they have been enrolled and randomized, when applicable.
If this is an open-label clinical trial, in which researchers and participants know which product or intervention is being administered, the patient will be informed which arm they were randomized to. If this is a double-blind study in which neither researchers nor participants know which product or intervention is being administered, the patient will not be informed which arm they were randomized to.
How can sites and sponsors improve pre-screening and screening processes to increase enrollment in clinical trials?
Because the screening pipeline determines which patients actually enroll in a trial, it’s critical to ensure that eligible patients aren’t falling through the cracks during – and leading up to – this phase. Earlier (and secure) data-sharing between sites and their sponsors on a digital platform can speed up the identification of factors affecting successful enrollment such as:
- Patterns in high screen failures. If data from certain sites is showing particularly high screen failure rates, the sponsor can consider reeducating those teams on protocol needs, especially if that protocol is complex.
- Insufficient representation across minorities. Site teams can collect patient race and ethnicity information as they move through pre-screening. With this data, it’s easier for both sites and sponsors to get an early understanding of the diversity picture of their clinical trial – are they moving a representative patient population into screening? Or are certain patient populations being disproportionately affected by certain I/E criteria before their screening visits? This information can inform possible updates to I/E criteria where safe and appropriate, and it can even inform possible updates to recruitment strategies earlier in the enrollment pipeline.
- Patterns in reasons patients decline to participate in the trial. When site teams work with patients who actively choose to leave the recruitment process, they can collect the specific reasons patients are declining participation prior to screening. Reasons can include “disinterested,” “too burdensome,” “too risky,” etc. If sponsors are able to access those reasons as they’re being collected, they can take corrective action to drive more eligible patients into screening. Perhaps they can improve patient education to boost interest, or they can provide transportation support to reduce perceived burden.
Screening and enrollment are different – but critical – factors in trial participation.
Without passing screening, a patient can’t successfully enroll in a clinical trial. Without successfully enrolling in a trial, a patient can’t receive an investigational product or intervention. When sites and sponsors work to keep these processes on track to meet diversity goals, to enroll eligible patients in a timely manner, and to prevent eligible patients from slipping through the cracks, all stakeholders can contribute to the advancement of therapeutic development.
Related Posts

How Does a Trial Manager in Greece Improve Clinical Trial Operations with StudyTeam®?
Dimitris Tziogas, local trial manager at a biotechnology company in ...
Read More
How to Address Key Clinical Trial Challenges, According to Clinresco Centres in South Africa
There’s no single solution to overcoming a research site’s specific ...
Read More
3 Clinical Trial Billing Challenges Research Sites Solve with StudyTeam
Challenge 1: Complicated coverage analysis Challenge 2: Tedious budgeting ...
Read More
.png?width=64&name=OST%20Transparent%20(1).png)