February 6th, 2024
Research Sites Can Now Use StudyTeam Technology to Enroll Clinical Trials in China
By OneStudyTeam
.png)
Research sites in China can now use StudyTeam software to manage patient recruitment and enrollment in clinical trials more efficiently.
Many of our sponsor partners have a strong presence in China, where they are running a large number of clinical trials. More are on the way. As a result, a growing number of partners have expressed a need to activate their sites in China on the StudyTeam platform.
Now, sponsors can do just that. Using StudyTeam for Sites, research site teams in China are empowered to collaborate with their sponsors while acting as the data controller. Once they log in, they can use tools within StudyTeam to improve patient scheduling, visit management, and overall workflows, while determining what patient information to enter into the platform. Site users in China can use the application translated into Simplified Mandarin. This language update improves each user’s understanding of patient data and visit management steps, while enabling clear communication across the whole team. Additionally, site users can navigate the platform, input data, and track enrollment progress more seamlessly, improving efficiency and reducing the risk of errors.
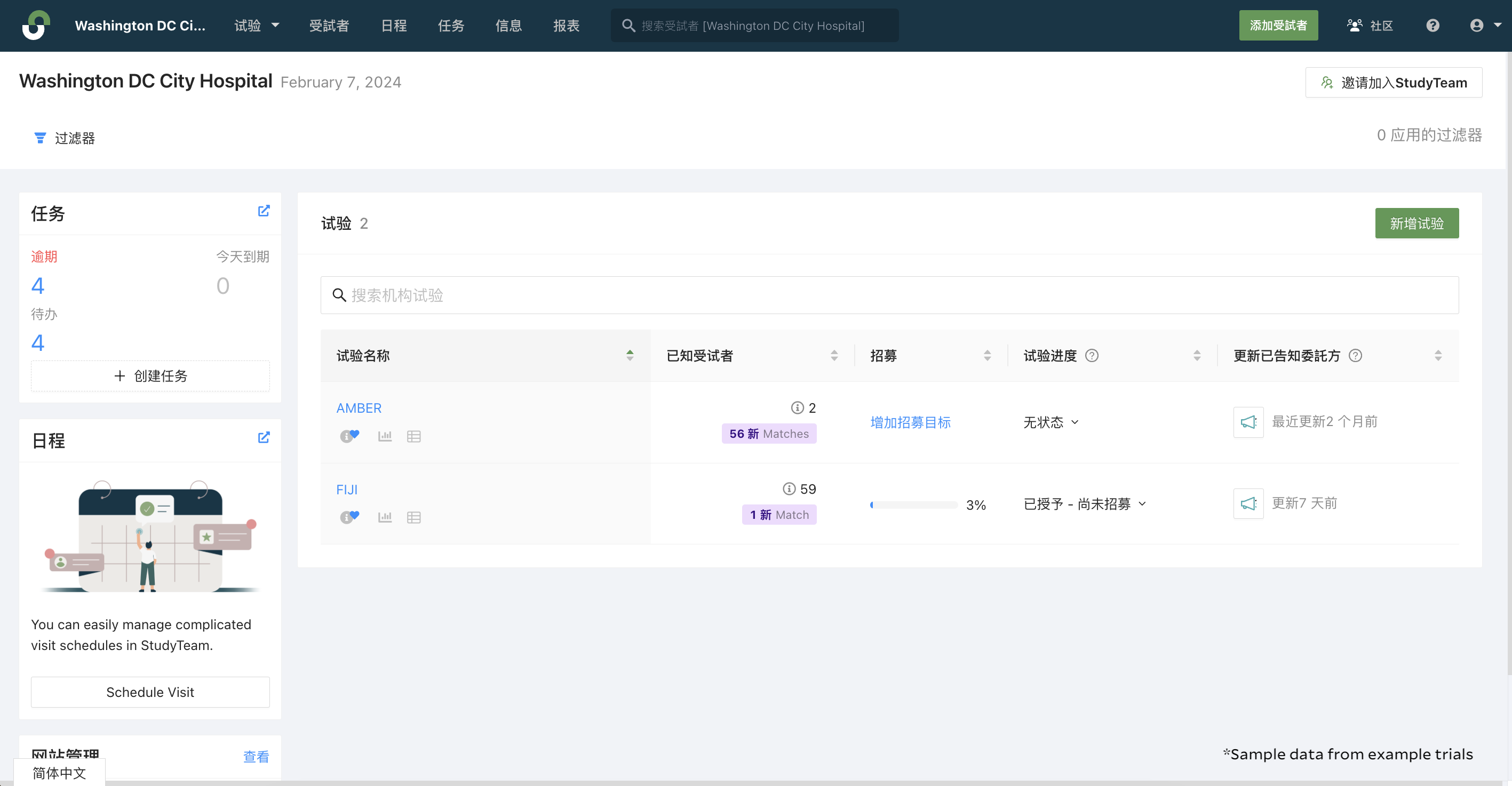
A de-identified version of site-entered information flows into StudyTeam for Sponsors, where the connected sponsor on the trial can view this data to identify barriers to patient recruitment and enrollment. This enables each sponsor to better support their site teams.

How is StudyTeam compliant with the regulatory landscape in China?
StudyTeam is compliant with the Personal Information Protection Law (PIPL) and the overall regulatory landscape in China.
Multiple regulations govern StudyTeam operations in China, including:
- the Population Health Information Regulation;
- the Big Data Regulation;
- the Regulation on the Management of Human Genetic Resources;
- the Detailed Implementation Rules for the Management Regulations of Human Genetic Resources;
- the Cybersecurity Law;
- the Data Security Law;
- the Personal Information Protection Law (PIPL).
The regulations form a backdrop for the types of data that can be collected, how they are to be protected and processed, and where the data can be stored.
As of July 2023, more than 4,000 sites outside of the United States use StudyTeam. In total, more than 7,000 sites use StudyTeam in more than 100 countries, including 93 percent of the European Union, 76 percent of Latin America, and 35 percent of countries in the Asia-Pacific region.
Related Posts

How Does a Trial Manager in Greece Improve Clinical Trial Operations with StudyTeam®?
Dimitris Tziogas, local trial manager at a biotechnology company in ...
Read More
How to Address Key Clinical Trial Challenges, According to Clinresco Centres in South Africa
There’s no single solution to overcoming a research site’s specific ...
Read More
3 Clinical Trial Billing Challenges Research Sites Solve with StudyTeam
Challenge 1: Complicated coverage analysis Challenge 2: Tedious budgeting ...
Read More
.png?width=64&name=OST%20Transparent%20(1).png)