April 4th, 2023
Strategies to Optimize Clinical Trial Enrollment Timelines
By OneStudyTeam
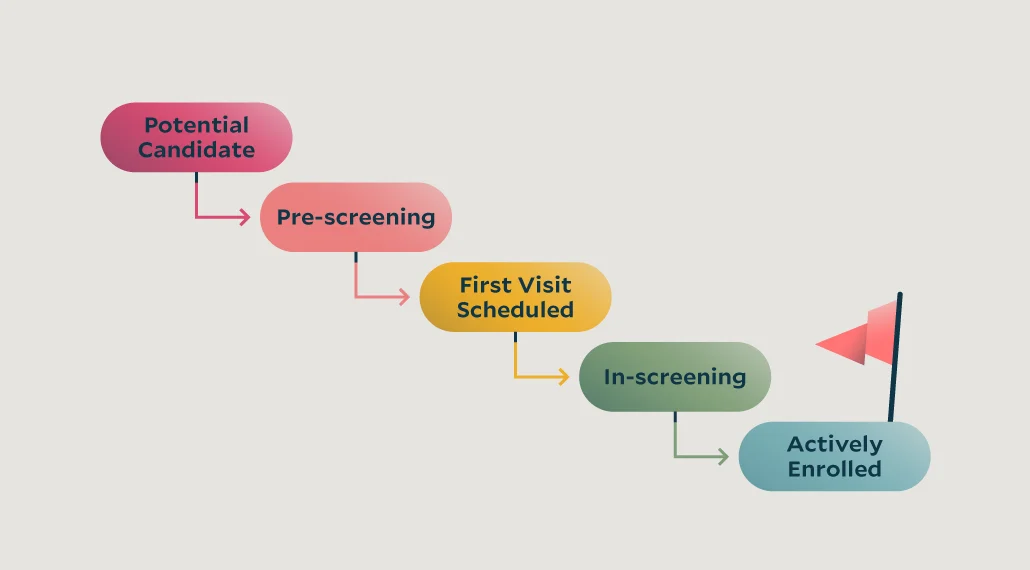
Patient enrollment in clinical trials is a major bottleneck in research — but it doesn’t have to be. That’s where patient enrollment optimization comes in.
What is patient enrollment optimization in clinical trials?
Patient enrollment optimization is a process by which sponsors use data to identify and remove barriers to enrollment before a trial is underway, and then again as enrollment moves forward. The process smooths out and accelerates the recruitment and enrollment of trial participants. The results? Reduced site burden, reduced sponsor costs, faster enrollment timelines, and the ability to get therapies to patients sooner.
Want to proactively address enrollment challenges across various stages of the clinical trial process? Start by focusing on these four steps:
- Analyze your protocol
- Analyze your sites’ performance history
- Implement pre-screening practices and tools
- Address rescreening opportunities in your protocol
By gathering insights during all of these stages and by taking action based on those insights, sponsors can optimize their clinical trial enrollment timelines. This not only benefits current trials, but sets a standard of best practices for all future trials.
Follow these steps as your initial checklist to make patient enrollment for clinical trials faster and more efficient.
/writing-better-clinical-trial-protocol-compressed.webp?width=466&height=258&name=writing-better-clinical-trial-protocol-compressed.webp)
Analyze your protocol
What is protocol feasibility? It’s the degree of possibility that your protocol can be successfully carried out according to plan. Not only is it helpful for sponsors to ensure that protocols are fairly straightforward for site teams to carry out on time, but it’s essential that protocols also incorporate clear and specific language so that deviations are unlikely across site locations.
Here are a few questions to ask when analyzing protocol feasibility:
- Can certain inclusion/exclusion criteria be more flexible to improve enrollment rates?
- Can patient burden be reduced around screening procedures to keep patients moving through the enrollment funnel?
- Is there room to expand your list of sites to reduce competition?
- Does the geographical area of the trial reach enough relevant patient populations?
Consider protocol feasibility from as many angles as possible.
Analyze your sites’ performance history
Gather performance metrics on clinical trial sites to better inform trial design and to anticipate opportunities for intervention or further training. Consider historical metrics including:
- Enrollment rates
- Participant retention rates
- Years of experience in the relevant therapeutic area
- Protocol deviations
Based on this information, you can take actionable steps to support better site performance. Could budgeting for a recruitment campaign drive more potential candidates into the enrollment funnel for better enrollment rates? Could investing in enrollment technology with visit window tolerances built in reduce the risk of protocol deviations?
Implement clinical trial pre-screening processes and tools
Pre-screening patients for clinical trial enrollment is a simple concept: site teams ask protocol-related questions of potential candidates while determining initial eligibility, then input that information into patient profiles. But site teams also need digital tools to simplify this process. For example, the StudyTeam performance enrollment management platform is programmed with pre-screening fields, according to protocol specifications, for sites to fill out as part of their typical workflows.
/track-prescreening-data-compressed.webp?width=394&height=218&name=track-prescreening-data-compressed.webp) With a digital pre-screening process in place:
With a digital pre-screening process in place:
- Sites build out informative patient databases to use across their trials
- Sponsors gain access to enrollment performance insights to inform protocol amendments, recruitment strategies, intervention opportunities, and more.
Thanks to the insights this practice generates, the pre-screening process in clinical trials is valuable even when trial enrollment is on track.
Address high screen failure rates
If your sites are struggling to enroll enough eligible patients for a trial, consult your enrollment data to determine whether screen failure rates seem too steep based on the number of patients who pass pre-screening. Because pre-screening questions are based on I/E criteria, patients are more likely to be eligible for trial enrollment. If very few of those patients are passing screening tests, it’s possible that sponsor intervention is necessary.
What to consider:
- Do site teams need further education on a complex protocol to ensure they fully comprehend the I/E criteria?
- Is a certain I/E criterion too rigid or possibly outdated – and can it be safely revised?
- Is rescreening a possibility for certain patients, and is that included as an option in the protocol?
See where the screening process can be updated, whether by site or sponsor teams, to reduce screen failures across your clinical trials.
Optimize patient enrollment timelines in your clinical trials
When sponsors roll out clinical trial enrollment strategies to proactively address challenges across various stages of their trials, they can eliminate barriers to enrollment for current and future trials. Analyze protocols, analyze site performance, track pre-screening data, and track reasons for screen failures to inform decisions that can improve enrollment numbers – and timelines – across your sites.
Related Posts

How Does a Trial Manager in Greece Improve Clinical Trial Operations with StudyTeam®?
Dimitris Tziogas, local trial manager at a biotechnology company in ...
Read More
How to Address Key Clinical Trial Challenges, According to Clinresco Centres in South Africa
There’s no single solution to overcoming a research site’s specific ...
Read More
3 Clinical Trial Billing Challenges Research Sites Solve with StudyTeam
Challenge 1: Complicated coverage analysis Challenge 2: Tedious budgeting ...
Read More

.png?width=64&name=OST%20Transparent%20(1).png)