December 7th, 2023
4 Ways StudyTeam’s eSource Solution Streamlines Site Workflows in Clinical Trials
By OneStudyTeam

The FDA’s 2013 guidance for electronic source data capture promotes the use of eSource in clinical trials to streamline investigations while improving the reliability, quality, integrity, and traceability of data. eSource platforms enable sites to capture all or some data for patient visits electronically, including medication logs.
How are sites choosing which eSource vendors to work with? It’s important to consider: Am I gaining an eSource platform that adds accuracy and ease to my clinical trial workflows? Here are four ways StudyTeam® for Sites’ eSource solution streamlines site workflows in clinical trials.
(1) Electronic data capture in StudyTeam can improve data quality.
Precise data entry ensures that trial findings accurately reflect the effect of a study on patients, so researchers and regulators alike can make informed decisions when bringing therapies to market. Precise data entry also prevents sites from doing extra work – you don’t have to follow up on missed lab assessments or data-entry errors.
To improve data quality, sites who use StudyTeam’s eSource solution are equipped with templates for electronic data capture that are designed to accurately align with each specific protocol and case report form.
To ensure accuracy, StudyTeam’s eSource functionality also includes validation. With eSource, you’re able to set ranges in certain fields to help prevent keystroke errors. For example, you can set an appropriate range for pulse rate, so that field is flagged if an improbable pulse rate is entered, to reduce errors.
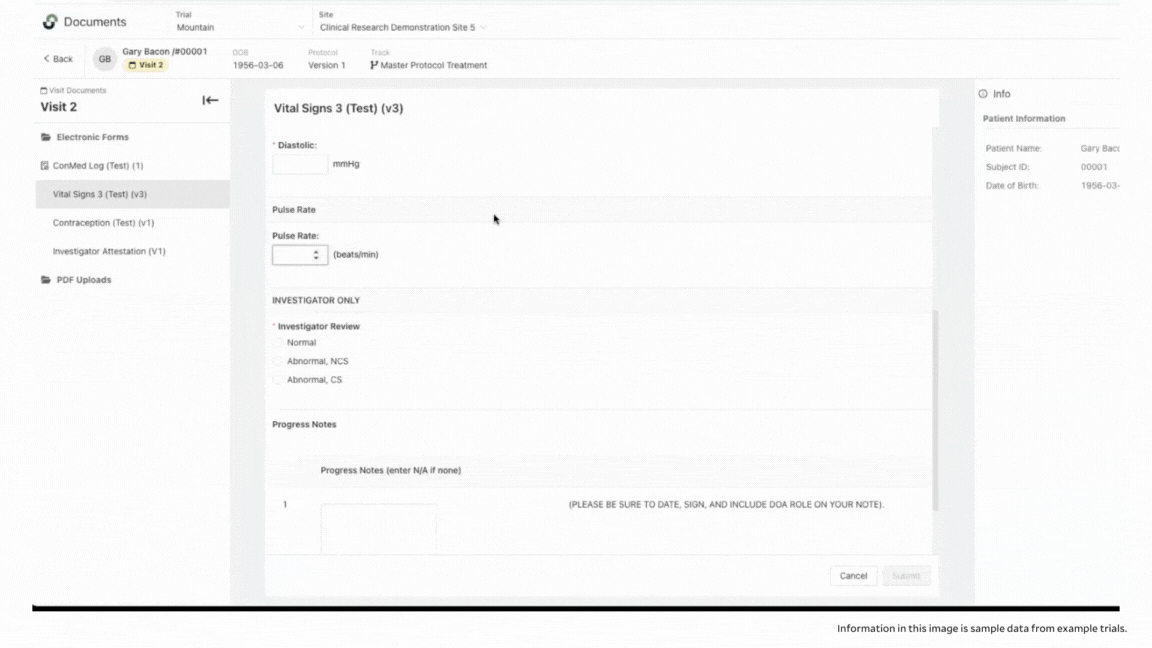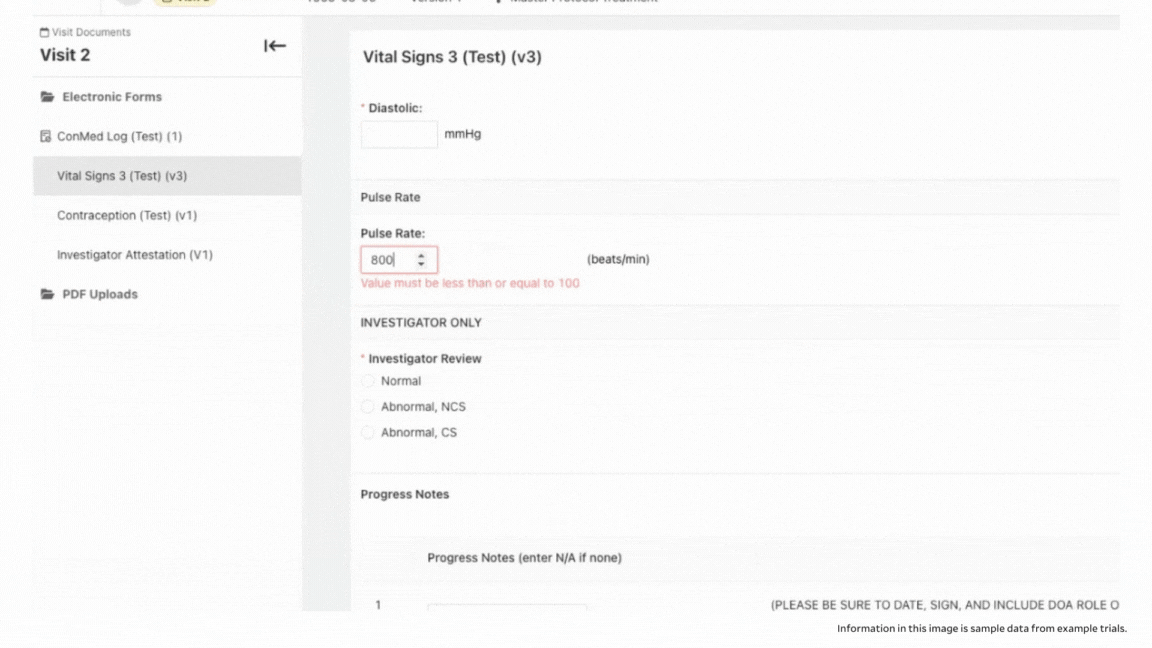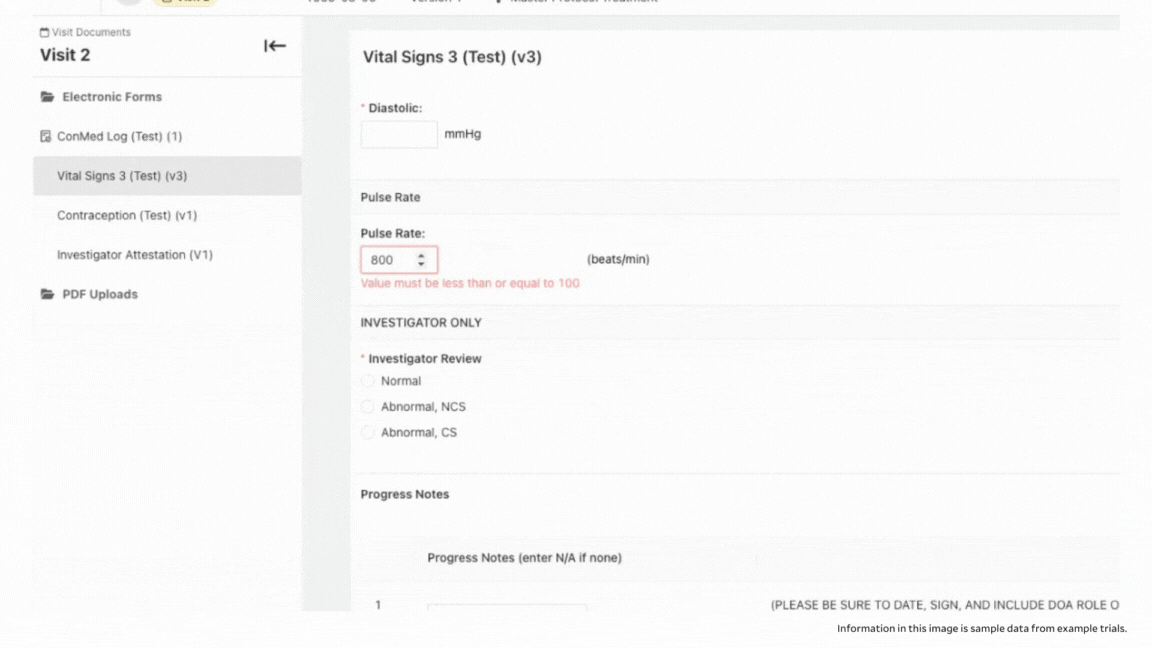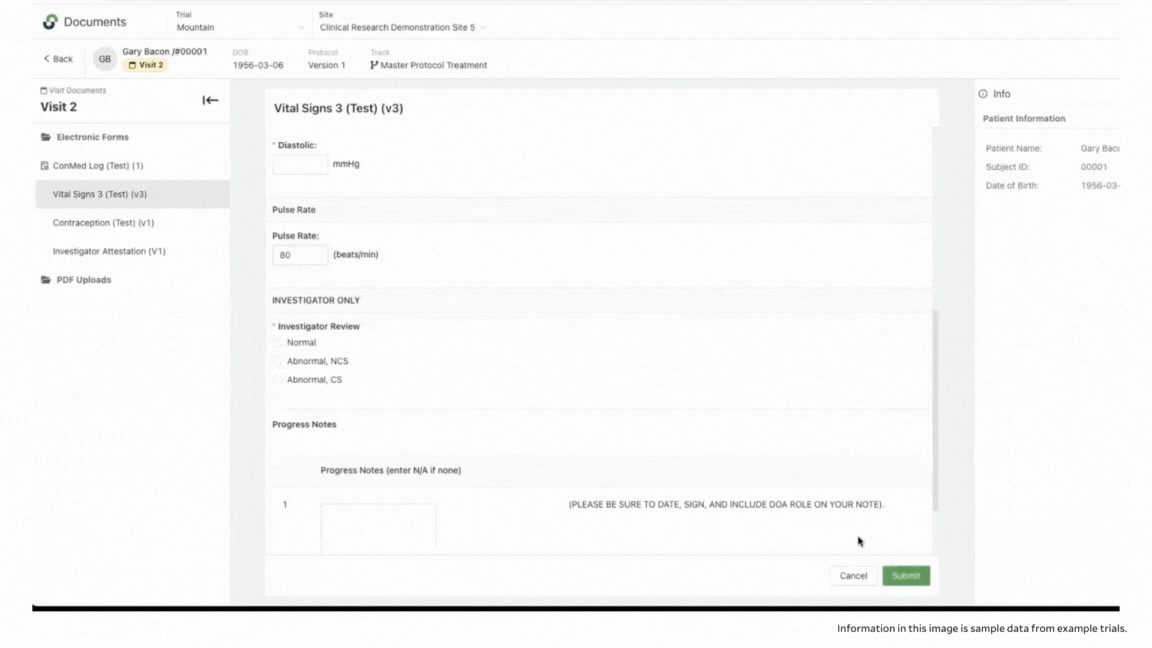
Another example is if a legitimate reading, like blood pressure, falls outside of an acceptable range per the protocol, you can be alerted and take appropriate action. This could qualify as an adverse event that needs to be documented, or it could require dropping the patient from the trial because they no longer meet the I/E criteria.
(2) With eSource in StudyTeam, sites save time and effort.
When you use StudyTeam to manage recruitment, enrollment, and ongoing patient visits, you can receive custom eSource forms designed for you, based on your trial’s protocol and your site’s custom needs. Receiving source templates that are already designed and configured saves you meaningful time.
You can then easily enter information into the digital forms with a few clicks and keystrokes while you manage patients. Because digital data entry improves both accuracy and legibility, you don’t have to spend time and effort correcting mistakes. You also prevent a slowdown in source management later on. To ensure you’re securely entering data, detailed standard operating procedures have been developed to ensure StudyTeam’s eSource capabilities are validated and in compliance with applicable regulations such as the FDA’s 21 CFR Part 11.
For sites who also prefer to use paper forms for certain processes, StudyTeam helps generate paper source templates with custom QR codes. When those paper forms are filled out and ready for upload, you can quickly upload multiple documents at once into a digital patient binder, with automatic categorization.
(3) Sites who use eSource in StudyTeam increase data completeness.
When you work with paper, it’s possible to inadvertently overlook a section of a form that needs to be filled out, especially when you’re overloaded with patients and complex protocols. With eSource in StudyTeam, you get additional guidance to ensure that study procedures are performed correctly. You actually see a visual representation of progress with each form: Progress bars indicate whether documentation for a screening visit, for example, is fully complete. StudyTeam users can see blue bars indicating incomplete documentation with an associated percentage of how close the tasks are to being completed; you can see green bars with a check mark indicating a visit that is 100% complete with all source documentation uploaded and ready to go.
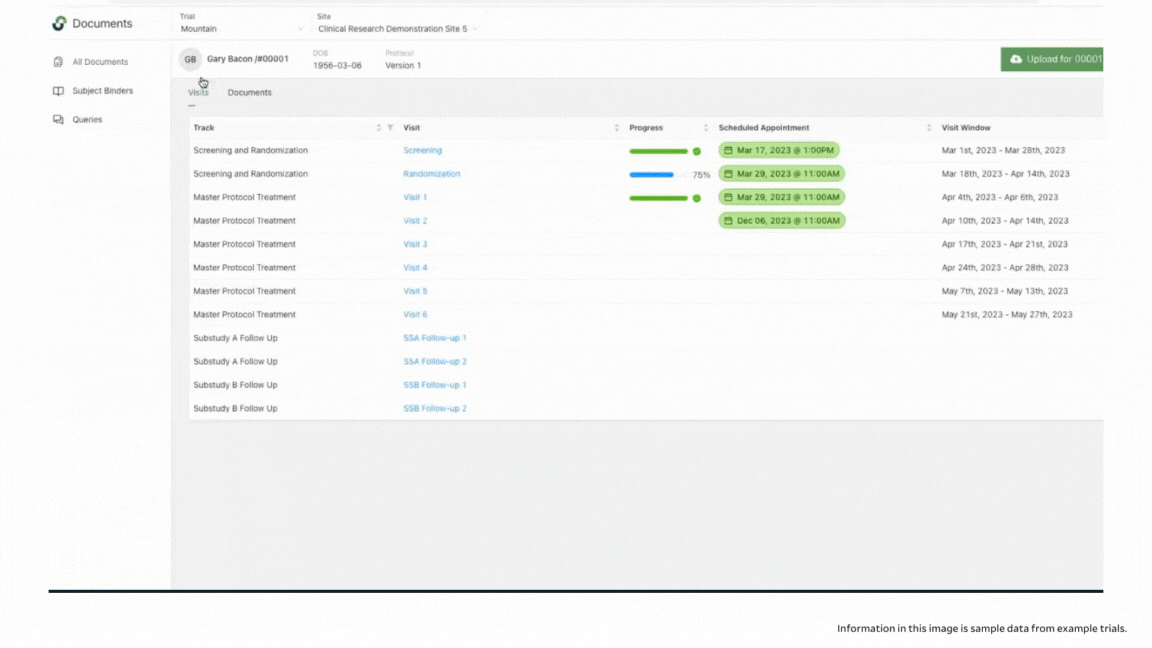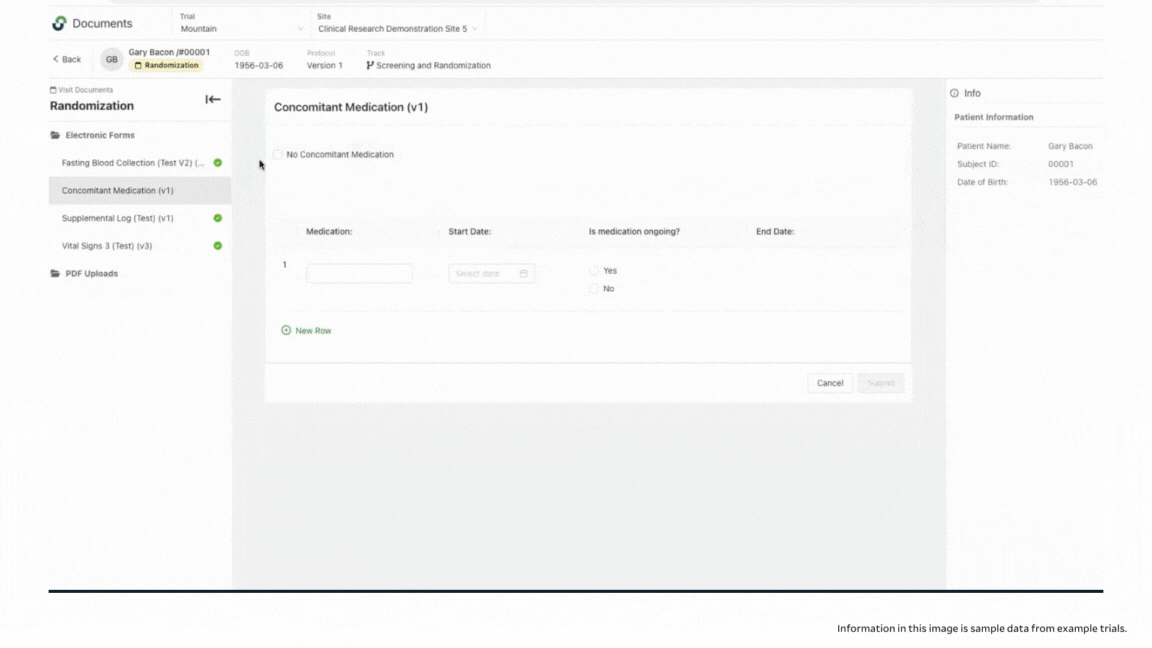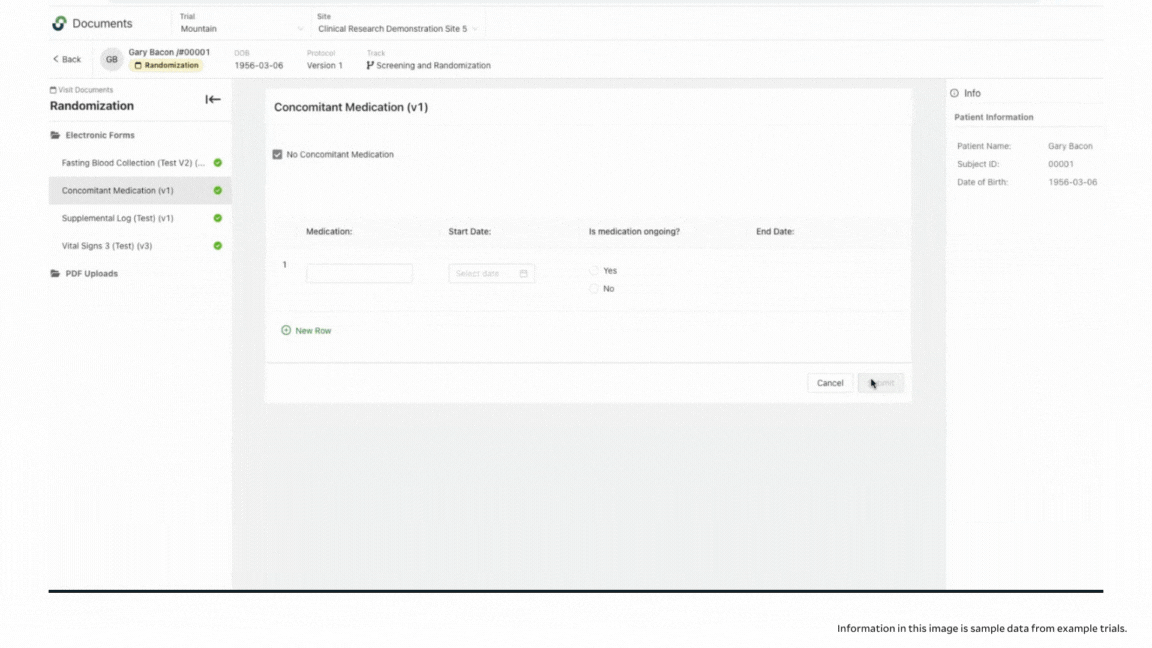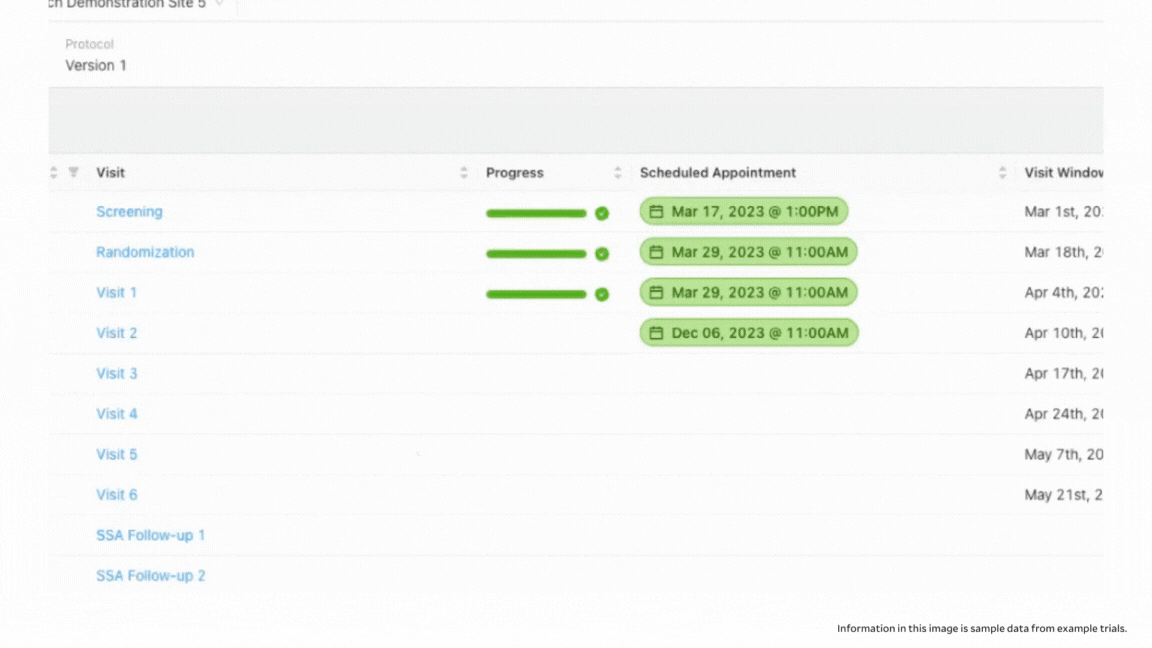
When you click into the incomplete visit, you can see which documents are completed in the patient binder, and which need additional information. If you are working with a paper document, you might never notice missing fields until you go to enter the information into your EDC, or until you get a query from your CRA. In addition to seeing completion levels, you can access a full audit trail to see who was in the system and who signed off on the work.
(4) StudyTeam’s eSource capabilities include expert technical support.
When you use the eSource solution in StudyTeam, you closely collaborate with a dedicated team of support professionals from our Site Success and Site Adoption teams. Our end goal is not only to provide you with software, but a solution with dedicated support to help you achieve your clinical trial objectives and help you achieve long-term success.
Our support professionals:
- Onboard and train new StudyTeam for Sites users;
- Manage the customization of eSource templates per trial;
- Process product feedback and enhancement requests;
- Provide ongoing support to StudyTeam for Sites users;
- Provide support with Standard Operating Procedures (SOPs).
“StudyTeam has been exceptionally responsive, providing invaluable support to our site’s research staff,” said Violet Heard, clinical research coordinator at Sound Medical Research in Port Orchard, Washington. “Moreover, the team at OneStudyTeam has consistently demonstrated a friendly and professional approach, always eager to seek and incorporate feedback. We genuinely appreciate the level of support StudyTeam delivers.”
Considering eSource vendors? Consider OneStudyTeam.
For sites that already use StudyTeam and its built-in Visit Schedule feature, it’s easy to implement our eSource solution. This doesn’t require an instant switch to digital-only source documentation; rather, eSource in StudyTeam allows you to bridge that process. If you choose to, you can slowly transition over time to digital source documentation without unnecessarily disrupting your workflow.
When eSource is used in conjunction with StudyTeam for Sites, research sites are empowered to streamline their clinical enrollment processes with higher accuracy, improved efficiency, and a reduced risk of protocol deviations and audit findings.
Related Posts
How Does a Trial Manager in Greece Improve Clinical Trial Operations with StudyTeam®?
Dimitris Tziogas, local trial manager at a biotechnology company in ...
Read More
How to Address Key Clinical Trial Challenges, According to Clinresco Centres in South Africa
There’s no single solution to overcoming a research site’s specific ...
Read More
3 Clinical Trial Billing Challenges Research Sites Solve with StudyTeam
Challenge 1: Complicated coverage analysis Challenge 2: Tedious budgeting ...
Read More

.png?width=64&name=OST%20Transparent%20(1).png)