January 28th, 2022
Key Challenges Managing Top-of-Funnel Patient Recruitment
By Reify Health
.webp)
While the industry spends more than $44 billion on clinical trials, 90% of potential patients are lost before enrollment. This signals a huge opportunity to drive more effective processes.
Let’s dive into the top of the recruitment funnel—an aspect of clinical trials significantly affected by unnecessary operational and logistical problems. In this chapter, we’ll discuss:
- Where potential participants get identified
- How inefficiencies in site workflows limit enrollment success
- Why seamless and transparent communication between sponsors and sites can bridge the gap
Thinking quality over quantity in trial recruitment
Today, a staggering 80% of all clinical trials fail to meet enrollment objectives, with both sites and sponsors trapped in the inertia of inefficiency. Sites get stuck with administrative work that prevents them from successfully moving candidates through the recruitment and enrollment process. Meanwhile, sponsors invest heavily to find qualified candidates for their clinical trials but have little or no visibility into which referral sources result in enrolled patients.
So where does it go wrong? At the beginning of a clinical trial, sites and sponsors start the process of identifying and sourcing candidates. A common misconception is that there’s a lack of patients available for clinical trials. While this is true in some cases, such as rare disease trials, the primary challenge is that the process of managing identified candidates and moving them towards enrollment is inefficient, scattered, and unnecessarily cumbersome.
Traditional sources of identifying and recruiting participants:
- Site and clinic databases: Many trial participants come from pools of patients that have interacted with sites for previous trials or for routine care. Sites often sift through their own EMR/EHR systems, charts, and patient records to find these candidates.
- Provider referrals: Providers who stay informed of potential trial opportunities for their patients both within their own site and across broader professional networks often make valuable referrals. In fact, our data suggest that candidates referred by providers within or outside of the research site enroll at the highest rates.
- Site-driven campaigns: Sites themselves support local ad campaigns to reach potential candidates (bus ads, for example) throughout the general population.
- Sponsor recruitment channels: A variety of sources available to sponsors, including:
- Recruitment vendors, which are organizations dedicated to generating referrals
- Direct/centralized recruitment campaigns such as direct-to-patient advertising, patient-facing websites, etc.
- Patient self-identification: While possible, this is rare, considering that 75% of people are unaware of clinical studies as a treatment option.
Administrative bottlenecks plaguing site staff
The variety of referral sources creates a major administrative challenge as sites enter the patient intake and pre-screening phases (the second stage in the timeline below). Nurses and site coordinators have to track numerous candidates across disparate systems—from email reports to Excel exports to loose-leaf paper with handwritten notes scribbled in the margins.
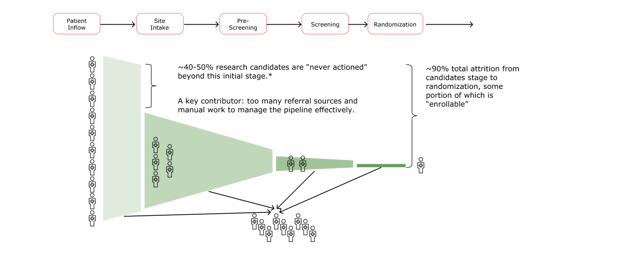
Beyond managing the massive number of inbound leads, clinical staff have to figure out which candidates are a match and begin pre-screening, a process that can become a juggling act. Sites contact potential and new patients individually to walk them through the clinical trial process and the trial’s procedures, determine if they’re a fit, follow up and make sure they’re feeling okay, schedule visits, ensure informed consent is complete, complete lab draws—all while simultaneously caring for patients, which includes more follow-up calls and scheduling visits. Given this inefficient referral system and the administrative fatigue it creates, it’s not surprising that between 40-50% of potential patients are lost in this initial stage of recruitment and enrollment.
Optimizing recruitment and enrollment at the top of the funnel allows both sites and sponsors to identify potential patients early on, create a system of visibility for all parties, and successfully and efficiently reach enrollment goals for clinical trials.
A need for transparency throughout the recruitment and enrollment process
By nature, clinical trials are at the forefront of therapeutic innovation and discovery, yet the process lags behind from a digital transformation standpoint. As illustrated below, a wealth of information from multiple sources comes together at the trial site, but traditional tracking methods lack efficiency and visibility, limiting the utility of the data. 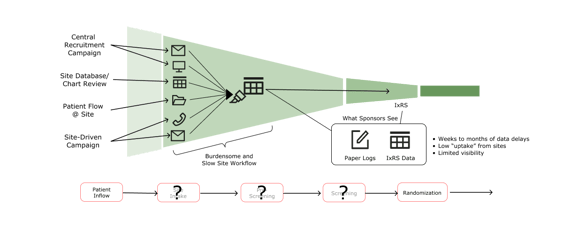
A win for sponsors, sites, and patients
Instead of manually tracking enrollment, cloud-based dashboards streamline the process and provide visibility for sites, sponsors, and partners to know what’s working and what’s not. This visibility helps clinical researchers make adjustments in near real-time while trials are enrolling.
Some insights coming from these data include:
- Differences in performance across study sites
- Differences in enrollment performance by the source of the candidates
- Disparities in gender, race/ethnicity, age, and other factors in recruited patients
- Inclusion/exclusion criteria that may be particularly limiting patient enrollment
- Limited reach in the patient recruitment pool
Sponsors and sites also benefit when they have a central database for future reference with longitudinal site data to inform relevant clinical trials. StudyTeam is committed to solving these specific problems through technology, as you can see in this case study.
More than half of recruited patients that are potentially eligible for a trial never get contacted or receive a follow-up from site staff; the patients are simply lost in the maze of recruitment work, according to analysis by StudyTeam. However, sites that use sponsor-connected enrollment applications move more than 60% of eligible patients through the recruitment and enrollment funnel.
Managing patient identification and engagement is just part of the top of the funnel, but it impacts the entire process and creates significant blind spots and pitfalls for the following stages of clinical trial development.
Learn more about solving clinical trial enrollment challenges in our new ebook, Strategies to Fill Gaps in Patient Recruitment and Enrollment.
Related Posts
How Does a Trial Manager in Greece Improve Clinical Trial Operations with StudyTeam®?
Dimitris Tziogas, local trial manager at a biotechnology company in ...
Read More
How to Address Key Clinical Trial Challenges, According to Clinresco Centres in South Africa
There’s no single solution to overcoming a research site’s specific ...
Read More
3 Clinical Trial Billing Challenges Research Sites Solve with StudyTeam
Challenge 1: Complicated coverage analysis Challenge 2: Tedious budgeting ...
Read More
