February 22nd, 2022
Diversity in Clinical Trials: Moving from Insight to Action, Earlier in the Funnel
By Reify Health
Improving the diversity of clinical trial participation is one of the most important initiatives across the life sciences industry. But the nature of capturing and reporting demographic information has limited sponsors’ ability to take corrective action early enough in a research program to affect change. A new category of solutions has emerged to provide race and ethnicity data earlier in the enrollment process, unveiling insights into how a trial’s design—from site selection and inclusion/exclusion criteria to patient education—can affect candidates from varying backgrounds throughout enrollment.
In a panel discussion at the Summit for Clinical Ops Executives (SCOPE), we spoke with Eli Lilly and Company’s Natalie Cheung Rotelli, advisor on diversity in clinical trials, and Seagen’s Neha Shah Londoño, director of clinical trial diversity and inclusion, about how the largest sponsor companies in the world use new insights to create strategies that improve diversity, access, and equity across their trials. OneStudyTeam’s Kent Siri, VP of marketing and head of diversity initiatives, released novel data about differences between white and Black patient journeys in the enrollment funnel.
Here are some of the highlights from the panel at SCOPE, edited for readability.
What inspired you to focus on diversity as the focus of your career?
Neha Londoño, Seagen: My decision to make diversity a career focus involved a collision course of personal priorities and social justice work that intersected with my clinical trials career. I had incorporated diversity work throughout my career, but now—especially after broad awareness driven by the pandemic and social justice movements in 2020—I’m excited to see sponsors building whole programs and departments focused on diversity.
Natalie Cheung Rotelli, Lilly: I grew up as one of the few Asian people in a small town, and I felt called to action to do everything I could for minority populations from my position at a company that makes medicines for people. I have a statistics background, so I wanted to make data-driven decisions to benefit the minority community.
What effect has the pandemic and the broader movement around social injustices had on public perceptions of research, particularly in underserved communities?
Cheung Rotelli, Lilly: The industry is paying attention to the disproportionate impacts of COVID on minorities, which has led to more attention on other disease areas disproportionately affecting minorities. The path forward starts with acknowledging mistrust in the past and identifying barriers, and this leads us to a clinical trials industry that tailors trial designs to be more inclusive.
Londoño, Seagen: People now have more familiarity with words like “efficacy” or “adverse events,” and understanding that type of language lets people ask questions about the care they receive and what it means for their health.
Kent Siri, OneStudyTeam: Diversity in clinical trials has been an identified issue for a long time, but the conversation has evolved since the onset of the COVID-19 pandemic as well as the recent movements around social justice. This is not just a 2022 problem nor will it be a 2022 solution. The industry has to be prepared to do the work for years to come as we collectively identify tactics and levers to affect change.
[Check out these articles for recent historical context: Clinical Trials Still Don't Reflect The Diversity Of America (NPR, 2015); Diversity Is Severely Lacking Among Clinical Trial Participants—How Can We Solve This Problem? (Forbes, 2019); Study Finds Diversity in Clinical Trials Still Lacking (PharmaNewsIntel, 2021)]
What has the evolving conversation around diversity in clinical trials meant for how sponsors are responding?
Londoño, Seagen: Advancing diversity in clinical trials requires a cultural mindset and change for the industry, similar to the approach of precision medicine, since both involve specific, tailored approaches to hard problems.
What data or insights do you feel that—if sponsors had them today—could drive meaningful change?
Londoño, Seagen: I value the ability to assess earlier in the funnel along the patient journey where the gaps are. Study managers can analyze aggregated data around specific entry criteria through StudyTeam to decide where to pivot, and this process specifically helps decisions about biomarkers and lab assessments—certain biomarkers might be too complex or not prevalent in a certain community, which would unnecessarily exclude certain populations from studies.
Siri, OneStudyTeam: It starts with knowing what you don’t know—before you build a bridge, you have to measure the width and depth of the gap. You can’t solve a problem until you’re able to measure the problem. In many analyses of clinical trial diversity, a lot of data reflects enrollment outcomes, but insights from earlier in the enrollment funnel let you identify where the gaps really are.
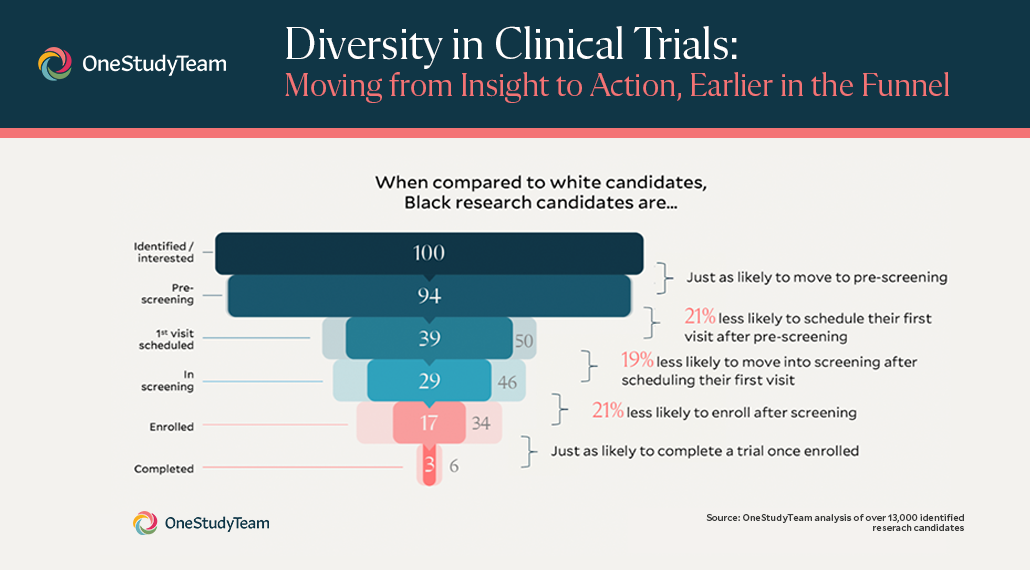
We looked at aggregated data from 13,000 identified research candidates. When compared to white candidates, Black research candidates are just as likely to move to pre-screening, 21% less likely to schedule their first visit after pre-screening, 19% less likely to move into screening after scheduling their first visit, 21% less likely to enroll after screening, and just as likely to complete a trial once enrolled.
.png?width=624&name=Untitled%20design%20(3).png)
Siri, OneStudyTeam: Moving from pre-screening to in-screening is where the process often moves from over-the-phone to in-person, involving certain operational barriers like transportation, child care, access to providers, and introducing informed consent to candidates.
How would someone in a role like yours use these types of data?
Cheung Rotelli, Lilly: Patient-level data like this [from OneStudyTeam] helps us understand the patient enrollment journey and identify the gaps. Patient- and site-level data gives sponsors the opportunity to reach out to sites and find ways to better support and partner with them to reach minority communities. It all starts with understanding what’s happening earlier in the patient journey.
How should the industry invest in repairing trust with minority communities?
Londoño, Seagen: Sponsors have a responsibility to listen and communicate transparently to patients. We have a responsibility to make long-term investments to benefit communities, including health literacy education, STEM education, resources for investigators of color, and broad education for people to understand how to explore clinical trials as a health care option.
What are some current initiatives you’re working on that you’re most excited about?
Cheung Rotelli, Lilly: I’m excited to shine light on areas we know less about to continue developing an understanding of the patient journey and applying it to clinical trials. That absolutely involves working within communities and through collaborative consortiums like TransCelerate. As we work with trusted intermediaries to engage with communities, we’re building foundations of health equity.
Learn more about how Reify Health helps sponsors advance diversity in clinical trials
Speaker bios
Neha Shah Londoño | Director, Clinical Trial Diversity and Inclusion, Seagen
Neha Londoño has twenty years industry experience within the sponsor, CRO, and biotech environment. She has extensive knowledge of the clinical trials process through career advancement, with a strong understanding of site and patient needs. Neha is currently leading the global clinical trial diversity and inclusion strategy at Seagen, with the objective of increasing and enhancing the representation of diverse populations, with a keen focus on improving the lives of all people with cancer.
Natalie Cheung Rotelli | Advisor on Diversity in Clinical Trials, Eli Lilly and Company
Natalie worked in non-profit and academia as a statistician before joining Lilly in 2006 as a statistical programmer. Her current role is advisor/director of diversity in clinical trials, leading their internal strategy. She received her BSPH and MPH in biostatistics from UNC, Chapel Hill and remains a rabid Tarheel fan despite her Midwest address. She enjoys cooking, running to counteract the cooking, and spending time with her two boys, one dog, and one husband.
Kent Siri | VP of Marketing and Head of Diversity Initiatives, OneStudyTeam
Kent is the vice president of marketing at OneStudyTeam where he oversees all functions related to the company’s corporate brand as well as OneStudyTeam's enrollment solutions. He also leads the organization’s diversity and inclusion efforts. Prior to joining OneStudyTeam, Kent led the brand team at Medidata. He received his bachelor’s degree in biology from Princeton and enjoys chasing children—preferably his own and ideally outside.
Related Posts

How Does a Trial Manager in Greece Improve Clinical Trial Operations with StudyTeam®?
Dimitris Tziogas, local trial manager at a biotechnology company in ...
Read More
How to Address Key Clinical Trial Challenges, According to Clinresco Centres in South Africa
There’s no single solution to overcoming a research site’s specific ...
Read More
3 Clinical Trial Billing Challenges Research Sites Solve with StudyTeam
Challenge 1: Complicated coverage analysis Challenge 2: Tedious budgeting ...
Read More
