June 3rd, 2021
What Does AstraZeneca’s Expansion of StudyTeam Mean for Sites?
By Reify Health Press Release
.webp)
We recently announced that AstraZeneca, one of the world’s leading biopharmaceutical companies, selected StudyTeam to accelerate clinical development across its breast cancer investigational therapy portfolio. One of AstraZeneca’s main objectives in choosing StudyTeam was to reinforce its reputation as a global sponsor of choice at breast cancer research sites.
We talked with Katherine Wall, senior data manager at clinical research site Redlands Hematology Oncology in Redlands, California, about what StudyTeam looks like in practice at one of the many research centers that AstraZeneca works with, and how StudyTeam helps sites succeed.
#1: StudyTeam creates a great overview of a site's patient population.
“I do weekly screenings, and I can open a screen and quickly see that a patient is entered into StudyTeam and a potential candidate for any of my studies. I can quickly confirm that a patient screening has been done and send it off to the study.”
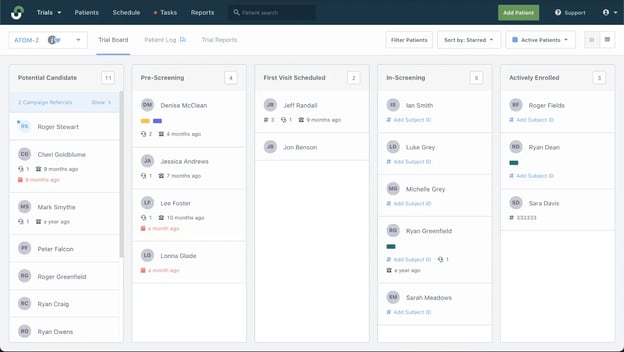
StudyTeam for Sites allows site staff to see and manage activities for all research candidates, across all stages of enrollment.
#2: Even when a sponsor isn't using StudyTeam for a trial yet, the sponsor's sites are using StudyTeam.
“We have a sponsor that doesn’t use StudyTeam yet for screening, and I still use StudyTeam to track patients so that I can know who we are watching, who is ineligible for the study, and who needs to be screened. That's a huge game changer for us. Since AstraZeneca introduced StudyTeam to us, we've just done our own thing with it, which is super helpful.
#3: StudyTeam simplifies inclusion/exclusion criteria for site staff.
“Study Team is just easier to navigate. Some of our patients have multiple disease states. With StudyTeam, when I enter the patient name, I know right away if they have conditions that would exclude them from a study. A spreadsheet wouldn’t do that for me.
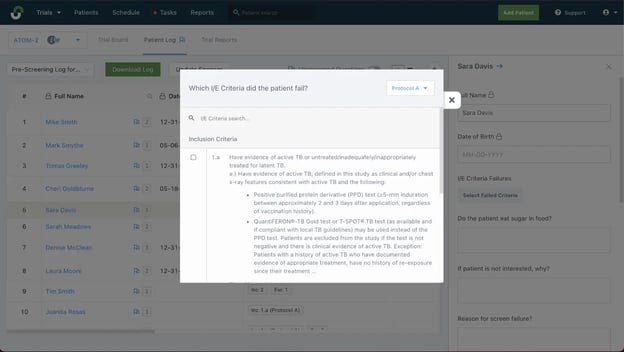
StudyTeam’s I/E criteria features allow for quick and systematic pre-screening.
Depending on the inclusion/exclusion criteria, a study can be hard to enroll, but StudyTeam makes the information I need readily available, which makes pre-screening easier and allows us to present potential patients to the principal investigator quickly.
#4: StudyTeam saves time for site staff.
“StudyTeam definitely saves me time. It's very easy and simple and streamlined. Before StudyTeam, I had to pull down a binder, pull the page out, and scan it for the information I need to send to the sponsor. These things add time to my day. But StudyTeam just makes my job easier.”
Since 2019, AstraZeneca has deployed StudyTeam across multiple large global oncology clinical trials. Sites using StudyTeam with AstraZeneca randomize more patients per site per month and provide overwhelmingly positive feedback; in fact, 100% of sites using StudyTeam in its trials choose to do so for subsequent studies.
Learn more about how sites are benefiting from StudyTeam in our Site Seeing series.
Related Posts

How Does a Trial Manager in Greece Improve Clinical Trial Operations with StudyTeam®?
Dimitris Tziogas, local trial manager at a biotechnology company in ...
Read More
How to Address Key Clinical Trial Challenges, According to Clinresco Centres in South Africa
There’s no single solution to overcoming a research site’s specific ...
Read More
3 Clinical Trial Billing Challenges Research Sites Solve with StudyTeam
Challenge 1: Complicated coverage analysis Challenge 2: Tedious budgeting ...
Read More
